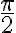Physics, Chemistry & Maths Glossary
Terminology and concepts related to Physics, Chemistry & Mathematics.
Browse the glossary using this index
A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ALL
W |
|---|
Walli's Formula | |
What is the role of asbestos in Wire Gauze?In short the answer is even distribution of heat. Bit more.... asbestos has ill health effects due to which ceramic is used these days. Both of these evenly distribute heat from the burner to the sample. | |
Why is Chloroform stored in closed dark coloured bottles?Chloroform is slowly oxidised by Oxygen present in air in the presence of light to Carbonyl Chloride (Phosgene) - an extremely poisonous gas - by the following reaction, \(2CHC{l_3} + {O_2}\xrightarrow{{light}}\mathop {2COC{l_2}}\limits_{Phosgene} + 2HCl\) By storing it in closed dark coloured bottles, this is prevented. | |